Polyarteritis Nodosa (PAN) Guidelines
2021 ACR/VF Guideline for the Management of Polyarteritis Nodosa (PAN)
These recommendations provide guidance regarding diagnostic strategies, use of pharmacologic agents, and imaging for patients with PAN.
Plain Language Recommendations
The ACR/VF Clinical Practice Guidelines were written for medical practitioners to help guide them in their treatment of patients with vasculitis. The plain language versions of these guidelines focus on the information that is most important for the majority of patients to know, and are written in language that can be more easily understood by those who do not have a medical background.
General Information
Sharon A. Chung,1 Mark Gorelik,2 Carol A. Langford,3 Mehrdad Maz,4![]() Andy Abril,5 Gordon Guyatt,6 Amy M. Archer,7 Doyt L. Conn,8
Andy Abril,5 Gordon Guyatt,6 Amy M. Archer,7 Doyt L. Conn,8![]() Kathy A. Full,9 Peter C. Grayson,10
Kathy A. Full,9 Peter C. Grayson,10![]() Maria F. Ibarra,11 Lisa F. Imundo,2 Susan Kim,1 Peter A. Merkel,12
Maria F. Ibarra,11 Lisa F. Imundo,2 Susan Kim,1 Peter A. Merkel,12![]() Rennie L. Rhee,12
Rennie L. Rhee,12![]() Philip Seo,13 John H. Stone,14
Philip Seo,13 John H. Stone,14![]() Sangeeta Sule,15
Sangeeta Sule,15![]() Robert P. Sundel,16 Omar I. Vitobaldi,17 Ann Warner,18 Kevin Byram,19 Anisha B. Dua,7 Nedaa Husainat,20
Robert P. Sundel,16 Omar I. Vitobaldi,17 Ann Warner,18 Kevin Byram,19 Anisha B. Dua,7 Nedaa Husainat,20![]() Karen E. James,21 Mohamad A. Kalot,22
Karen E. James,21 Mohamad A. Kalot,22![]() Yih Chang Lin,23 Jason M. Springer,4
Yih Chang Lin,23 Jason M. Springer,4![]() Marat Turgunbaev,24 Alexandra Villa-Forte,3 Amy S. Turner,24
Marat Turgunbaev,24 Alexandra Villa-Forte,3 Amy S. Turner,24 and Reem A. Mustafa25
and Reem A. Mustafa25![]()
Guidelines and recommendations developed and/or endorsed by the American College of Rheumatology
(ACR) are intended to provide guidance for particular patterns of practice and not to dictate the care of a particular patient. The ACR considers adherence to the recommendations within this guideline to be voluntary, with the ultimate determination regarding their application to be made by the physician in light of each patient’s individual circumstances. Guidelines and recommendations are intended to promote beneficial or desirable outcomes but cannot guarantee any specific outcome. Guidelines and recommendations developed and endorsed by the ACR are subject to periodic revision as warranted by the evolution of medical knowledge, technology, and practice. ACR recommendations are not intended to dictate payment or insurance decisions, and drug formularies or other third-party analyses that cite ACR guidelines should state this. These recommendations cannot adequately convey all uncertainties and nuances of patient care.
The American College of Rheumatology is an independent, professional, medical and scientific society that does not guarantee, warrant, or endorse any commercial product or service.
Objective. To provide evidence-based recommendations and expert guidance for the management of systemic polyarteritis nodosa (PAN).
Methods. Twenty-one clinical questions regarding diagnostic testing, treatment, and management were developed in the population, intervention, comparator, and outcome (PICO) format for systemic, non–hepatitis B–related PAN. Systematic literature reviews were conducted for each PICO question. The Grading of Recommendations Assessment, Development and Evaluation methodology was used to assess the quality of evidence and formulate recommendations. Each recommendation required ≥70% consensus among the Voting Panel.
Results. We present 16 recommendations and 1 ungraded position statement for PAN. Most recommendations were graded as conditional due to the paucity of evidence. These recommendations support early treatment of severe PAN with cyclophosphamide and glucocorticoids, limiting toxicity through minimizing long-term exposure to both treatments, and the use of imaging and tissue biopsy for disease diagnosis. These recommendations endorse minimizing risk to the patient by using established therapy at disease onset and identify new areas where adjunctive therapy may be warranted.
Conclusion. These recommendations provide guidance regarding diagnostic strategies, use of pharmacologic agents, and imaging for patients with PAN.
Polyarteritis nodosa (PAN) is a systemic necrotizing vasculitis that primarily affects medium-sized vessels (1). Patients frequently present with systemic symptoms such as fever and weight loss.
The most common clinical presentations include neurologic manifestations such as mononeuritis multiplex and peripheral neuropathy, cutaneous manifestations such as nodules and livedo reticularis, renal manifestations such as hypertension, and gastrointestinal manifestations such as abdominal pain (2). Diagnosis is generally confirmed by tissue biopsy of an affected organ or angiography if tissue biopsy cannot be obtained. Typical histologic findings include mixed-cell inflammatory infiltrates in the vessel wall and fibrinoid necrosis, with an absence of granulomas and giant cells (3). Findings on angiography include saccular or fusiform aneurysms and stenotic lesions in the mesenteric, hepatic, and renal arteries and their subsequent branches. Although PAN is becoming increasingly rare due to the prevention of hepatitis B viral (HBV) infection, it remains a potentially devastating diagnosis, with severe PAN having a mortality rate of 40% at 5 years (3).
Given the increasing options available to treat systemic vasculitis, the American College of Rheumatology (ACR) and the Vasculitis Foundation (VF) supported the development of guidelines for the management of large, medium, and small vessel vasculitis. This guideline presents evidence-based recommendations for the diagnostic testing, treatment, and management of PAN as an exemplar of medium vessel vasculitis. Of note, this guideline focuses on systemic PAN. Since HBV-associated PAN as well as cutaneous PAN are generally managed differently from systemic idiopathic PAN, they were excluded from this guideline.
Although this guideline may inform an international audience, these recommendations were developed considering the experience with and availability of treatment and diagnostic options in the US.
This guideline followed the ACR guideline development process
(https://www.rheumatology.org/Practice-Quality/Clinical-Support/Clinical-Practice-Guidelines) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to rate the quality of evidence and develop recommendations (4,5). ACR policy guided the management of conflicts of interest and disclosures (https://www.rheumatology.org/Practice-Quality/Clinical-Support/Clinical-Practice-Guidelines/Vasculitis). Supplementary Appendix 1 (available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41776/abstract) presents a detailed description of the methods. Briefly, the Literature Review team undertook systematic literature reviews for predetermined questions specifying the clinical population, intervention, comparator, and outcomes (PICO). An in-person Patient Panel of 11 individuals with different types of vasculitis (1 patient with PAN) was moderated by a member of the Literature Review team (ABD). This Patient Panel reviewed the evidence report (along with a summary and interpretation by the moderator) and provided patient perspectives and preferences. An Expert Panel provided expert knowledge to inform discussion of the PICO questions and findings of the literature review. The Voting Panel comprised 9 adult rheumatologists, 5 pediatric rheumatologists, and 2 patients; they reviewed the Literature Review team’s evidence summaries and, bearing in mind the Patient Panel’s deliberations, formulated and voted on recommendations. A recommendation required ≥70% consensus among the Voting Panel.
How to interpret the recommendations
A strong recommendation is typically supported by moderate to high-quality evidence trials). For a strong recommendation, the recommended course of action would apply to all or almost all patients. Only a small proportion of clinicians/patients would not want to follow the recommendation.
In rare instances, a strong recommendation may be based on very low– to low-certainty evidence. For example, an intervention may be strongly recommended if it is considered low-cost, without harms, and the consequence of not performing the intervention may be catastrophic. An intervention may be strongly recommended against if there is high certainty that the intervention leads to more harm than the comparison with very low or low certainty about its benefit (6).
A conditional recommendation is generally supported by lower-quality evidence or a close balance between desirable and undesirable outcomes. For a conditional recommendation, the recommended course of action would apply to the majority of the patients, but the alternative is a reasonable consideration. Conditional recommendations always warrant a shared decision-making approach. We specify conditions under which the alternative may be considered.
In some instances, the committee found that the evidence for a particular PICO question did not support a graded recommendation or did not favor one intervention over the other. However, the Voting Panel believed that the PICO question addressed a commonly encountered clinical question and thus felt that providing guidance for this question was warranted. For these situations, we present “ungraded position statements,” which reflect general views of the Voting Panel.
In this evidence-based guideline, we explicitly used the best evidence available and present that in a transparent manner for the clinician reader/user (7). In some instances, this includes randomized trials in which the interventions under consideration are directly compared. The GRADE system rates evidence that comes exclusively from the collective experience of the Voting Panel and Patient Panel members as “very low quality” evidence (5).
For each recommendation, details regarding the PICO questions and the GRADE evidence tables can be found in Supplementary Appendix 2 (http://onlinelibrary.wiley.com/doi/10.1002/art.41776/ abstract).
This is the first guideline issued by the ACR, in conjunction with the VF, for the management of systemic PAN. These recommendations constitute a guide to help physicians treat patients with this disease. Because many recommendations are conditional, a patient’s clinical condition, values, and preferences should influence the management decisions that are made. These recommendations should not be used by any agency to restrict access to therapy or require that certain therapies be utilized prior to other therapies.
Classic systemic PAN, although rare, remains a disease with a high mortality rate (22). Therefore, recommendations in this guideline indicate that patients with severe disease should be treated with cyclophosphamide and glucocorticoids. However, when patients present with nonsevere disease (i.e., without life-or organ-threatening manifestations such as renal insufficiency and tissue ischemia), use of alternative immunosuppressive agents and a glucocorticoid-sparing regimen is reasonable for remission induction. Use of diagnostic procedures such as angiography, electromyography/nerve conduction studies, and nerve and muscle biopsy is recommended to aid in diagnosis. However, the use of routinely repeated procedures during periods of disease quiescence is discouraged.
PAN has become increasingly rare, and no large clinical trials that focused solely on idiopathic (non–BV-associated) PAN have been published. In addition, studies of PAN conducted prior to the recognition of microscopic polyangiitis may have included such patients and should be interpreted with caution. Many recommendations were based on expert experience of the Voting Panel and/or trials that were performed several years and, in some cases, decades ago. Strong recommendations will require larger interventional studies but will be challenging to conduct due to the rarity of this disease.
The process of developing these guidelines has brought to our attention other gaps in our understanding of the optimal treatment for PAN. These gaps include the role of longitudinal vascular imaging studies, the comparative effectiveness of nonglucocorticoid immunosuppressive agents, and the lack of biomarkers to inform disease activity or treatment response. Therefore, we encourage continued research in this disease. Future study and specific areas to investigate include the following: 1) determining how informative longitudinal vascular imaging is for assessing disease activity and determining disease prognosis; 2) conducting randomized clinical trials (including comparative efficacy trials) to assess the efficacy of nonglucocorticoid immunosuppressive agents, as well as identifying the optimal dosing, duration, and population that would benefit from these agents; 3) developing novel, targeted, and/or glucocorticoid-sparing therapies with minimal toxicity; and 4) identifying biomarkers to inform assessment of disease activity and prognosis.
In summary, the ACR and the VF present these recommendations to assist physicians in managing PAN, and this guideline can serve as a touchstone for basic principles of management. We hope this guideline will evolve as new research is conducted and new diagnostic and treatment strategies for PAN are identified.
We thank Anne M. Ferris, MBBS, Ora Gewurz-Singer, MD, Rula Hajj-Ali, MD, Eric Matteson, MD, MPH, Robert F. Spiera, MD, Linda Wagner-Weiner, MD, MS, and Kenneth J. Warrington, MD, for serving on the Expert Panel. We thank Antoine G. Sreih, MD, and Gary S. Hoffman, MD, MS, for their contributions during the early phases of this project as members of the Core Team. Dr. Hoffman’s participation ended July 2018 due to personal reasons. Dr. Sreih’s involvement ended in December 2018 when he became primarily employed by industry, which precluded his continued participation in this project. We thank Joyce Kullman (Vasculitis Foundation) for her assistance with recruitment for the Patient Panel. We thank the patients who (along with authors Kathy A. Full and Omar I. Vitobaldi) participated in the Patient Panel meeting: Jane Ascroft, Scott A. Brunton, Dedra DeMarco, Thomas Fitzpatrick, Jenn Gordon, Maria S. Mckay, Sandra Nye, Stephanie Sakson, and Ben Wilson. We thank Robin Arnold, Catherine E. Najem, MD, MSCE, and Amit Aakash Shah, MD, MPH, for their assistance with the literature review. We thank the ACR staff, including Ms Regina Parker, for assistance in organizing the face-to-face meeting and coordinating the administrative aspects of the project, and Ms Robin Lane for assistance in manuscript preparation. We thank Ms Janet Waters for help in developing the literature search strategy and performing the initial literature search, and Ms Janet Joyce for performing the update searches.
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Chung and Gorelik had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Chung, Gorelik, Langford, Maz, Abril, Guyatt, Archer, Full, Grayson, Merkel, Seo, Stone, Sule, Sundel, Vitobaldi, Turner, Mustafa.
Acquisition of data. Chung, Gorelik, Langford, Maz, Abril, Full, Imundo, Kim, Merkel, Stone, Vitobaldi, Byram, Dua, Husainat, James, Kalot, Lin, Springer, Turgunbaev, Villa-Forte, Turner, Mustafa.
Analysis and interpretation of data. Chung, Gorelik, Langford, Maz, Abril, Archer, Conn, Full, Grayson, Ibarra, Imundo, Kim, Merkel, Rhee, Seo, Stone, Vitobaldi, Warner, Byram, Dua, Husainat, Kalot, Lin, Springer, Turgunbaev, Mustafa.
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 2013;65:1–11.
- Pagnoux C, Seror R, Henegar C, Mahr A, Cohen P, Le Guern V, et al. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group database. Arthritis Rheum 2010;62:616–26.
- Hernandez-Rodriguez J, Alba MA, Prieto-Gonzalez S, Cid MC. Diagnosis and classification of polyarteritis nodosa [review]. J Autoimmun 2014;48–49:84–9.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6.
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719–25.
- Alexander PE, Gionfriddo MR, Li SA, Bero L, Stoltzfus RJ, Neumann I, et al. A number of factors explain why WHO guideline developers make strong recommendations inconsistent with GRADE guidance. J Clin Epidemiol 2016;70:111–22.
- Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, et al. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach [review]. J Clin Epidemiol 2016;72:45–55.
- Hekali P, Kajander H, Pajari R, Stenman S, Somer T. Diagnostic significance of angiographically observed visceral aneurysms with regard to polyarteritis nodosa. Acta Radiol 1991;32:143–8.
- Pagnoux C, Mahr A, Cohen P, Guillevin L. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: analysis of 62 patients with polyarteritis nodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine (Baltimore) 2005;84:115–28.
- Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222–7.
- Stanson AW, Friese JL, Johnson CM, McKusick MA, Breen JF, Sabater EA, et al. Polyarteritis nodosa: spectrum of angiographic findings. Radiographics 2001;21:151–9.
- Singhal M, Gupta P, Sharma A, Lal A, Rathi M, Khandelwal N. Role of multidetector abdominal CT in the evaluation of abnormalities in polyarteritis nodosa. Clin Radiol 2016;71:222–7.
- Schmidt WA. Use of imaging studies in the diagnosis of vasculitis [review]. Curr Rheumatol Rep 2004;6:203–11.
- Pipitone N, Versari A, Salvarani C. Role of imaging studies in the diagnosis and follow-up of large-vessel vasculitis: an update. Rheumatology (Oxford) 2008;47:403–8.
- Albert DA, Silverstein MD, Paunicka K, Reddy G, Chang RW, Derus C. The diagnosis of polyarteritis nodosa. II. Empirical verification of a decision analysis model. Arthritis Rheum 1988;31:1128–34.
- Wees SJ, Sunwoo IN, Oh SJ. Sural nerve biopsy in systemic necrotizing vasculitis. Am J Med 1981;71:525–32.
- Walker GL. Neurological features of polyarteritis nodosa. Clin Exp Neurol 1978;15:237–47.
- Martinez AC, Barbado FJ, Ferrer MT, Vazquez JJ, Conde MC, Aquado AG. Electrophysiological study in systemic necrotizing vasculitis of the polyarteritis nodosa group. Electromyogr Clin Neurophysiol 1988;28:167–73.
- Vital C, Vital A, Canron MH, Jaffre A, Viallard JF, Ragnaud JM, et al. Combined nerve and muscle biopsy in the diagnosis of vasculitic neuropathy: a 16-year retrospective study of 202 cases. J Peripher Nerv Syst 2006;11:20–9.
- Ribi C, Cohen P, Pagnoux C, Mahr A, Arène JP, Puéchal X, et al. Treatment of polyarteritis nodosa and microscopic polyangiitis without poor-prognosis factors: a prospective randomized study of one hundred twenty-four patients. Arthritis Rheum 2010;62:1186–97.
- Samson M, Puéchal X, Mouthon L, Devilliers H, Cohen P, Bienvenu B, et al. Microscopic polyangiitis and non-HBV polyarteritis nodosa with poor-prognosis factors: 10-year results of the prospective CHUSPAN trial. Clin Exp Rheumatol 2017;35 Suppl 103:176–84.
- Samson M, Puechal X, Devilliers H, Ribi C, Cohen P, Bienvenu B, et al. Long-term follow-up of a randomized trial on 118 patients with polyarteritis nodosa or microscopic polyangiitis without poor-prognosis factors [review]. Autoimmun Rev 2014;13:197–205.
- Lipworth BJ. Therapeutic implications of non-genomic glucocorticoid activity [editorial]. Lancet 2000;356:87–9.
- Ruiz-Irastorza G, Ugarte A, Terrier CS, Lazaro E, Iza A, Couzi L, et al. Repeated pulses of methyl-prednisolone with reduced doses of prednisone improve the outcome of class III, IV and V lupus nephritis: an observational comparative study of the Lupus-Cruces and lupus-Bordeaux cohorts [review]. Autoimmun Rev 2017;16:826–32.
- Cohen RD, Conn DL, Ilstrup DM. Clinical features, prognosis, and response to treatment in polyarteritis. Mayo Clin Proc 1980;55:146–55.
- Gayraud M, Guillevin L, le Toumelin P, Cohen P, Lhote F, Casassus P, et al. Long-term followup of polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: analysis of four prospective trials including 278 patients. Arthritis Rheum 2001;44:666–75.
- Mina R, von Scheven E, Ardoin SP, Eberhard BA, Punaro M, Ilowite N, et al. Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012;64:375–83.
- Seri Y, Shoda H, Hanata N, Nagafuchi Y, Sumitomo S, Fujio K, et al. A case of refractory polyarteritis nodosa successfully treated with rituximab. Mod Rheumatol 2017;27:696–8.
- Ribeiro E, Cressend T, Duffau P, Grenouillet-Delacre M, Rouanet-Larivière M, Vital A, et al. Rituximab efficacy during a refractory polyarteritis nodosa flare. Case Rep Med 2009;738293.
- Al-Homood IA, Aljahlan MA. Successful use of combined corticosteroids and rituximab in a patient with refractory cutaneous polyarteritis nodosa. J Dermatol Dermatol Surg 2017;21:24–6.
- Leib ES, Restivo C, Paulus HE. Immunosuppressive and corticosteroid therapy of polyarteritis nodosa. Am J Med 1979;67:941–7.
- Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, le Toumelin P, for the French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011;90:19–27.
- Guillevin L, Lhote F, Cohen P, Jarrousse B, Lortholary O, Généreau T, et al. Corticosteroids plus pulse cyclophosphamide and plasma exchanges versus corticosteroids plus pulse cyclophosphamide alone in the treatment of polyarteritis nodosa and Churg-Strauss syndrome patients with factors predicting poor prognosis: a prospective, randomized trial in sixty-two patients. Arthritis Rheum 1995;38:1638–45.
- Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377:317–28.
- Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zaviolov AV, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med 2014;370:911–20.
- Schnappauf O, Moura NS, Aksentijevich I, Stoffels M, Ombrello AK, Hoffmann P, et al. Sequence-based screening of patients with idiopathic polyarteritis nodosa, granulomatosis with polyangiitis, and microscopic polyangiitis for deleterious genetic variants in ADA2. Arthritis Rheumatol 2020;73:512–9.
- Navon Elkan P, Pierce SB, Segel R, Walsh T, Barash J, Padeh S, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med 2014;370:921–31.
Results
For the evidence report, the Literature Review team summarized 127 articles to address 21 PICO questions for PAN.
The following recommendations and ungraded position statements are for systemic PAN and do not apply to isolated cutaneous or HBV-related PAN.
Recommendations and Positions for PAN
Table 1 presents definitions of selected terms used in the recommendations, including the definition of severe and nonsevere disease, as well as dosing ranges for glucocorticoids. Table 2 presents the recommendations with their supporting PICO questions and levels of evidence. Figure 1 provides key recommendations for the treatment for PAN. All but 1 of the recommendations are conditional, primarily due to lack of high-quality evidence (e.g., randomized controlled trials) supporting the recommendation.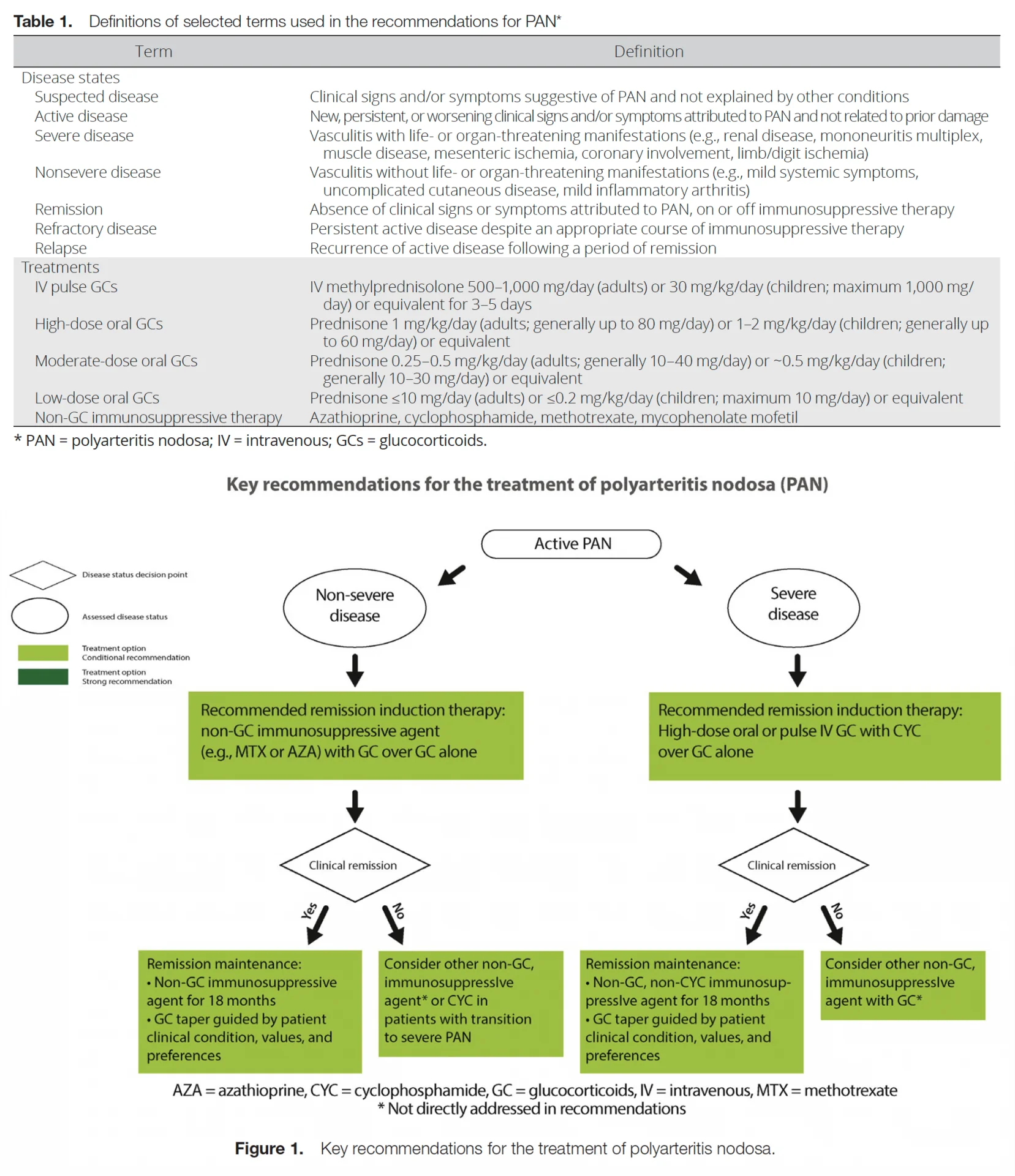
Recommendation: For patients with suspected PAN, we conditionally recommend using abdominal vascular imaging to aid in establishing a diagnosis and determining the extent of disease.
Evidence for the use of routine diagnostic imaging is limited, with no comparative trials available. In single-arm studies that were performed when diagnostic criteria for PAN were not well defined, vascular imaging, in tandem with clinical signs and pathology, helped validate the diagnosis (8) and determine disease severity (9). This in turn can influence treatment decisions. Moreover, obtaining vascular imaging at disease onset facilitates identification of new vascular involvement during disease relapse. Vascular imaging may not be warranted if patients present with isolated findings such as mononeuritis multiplex or myopathy, or if there are no clinical features suggestive of abdominal arterial involvement (such as absence of gastrointestinal or genitourinary symptoms, including renovascular hypertension). For children, clinicians should be mindful of minimizing repeated radiation exposure.
Clinicians currently use both conventional catheter-based dye angiography and noninvasive methods such as computed tomography (CT) or magnetic resonance (MR) angiography to diagnose PAN (10–12).
Conventional angiography is the current gold standard due to its ability to provide better resolution, but it can be associated with complications, albeit at a very low rate (13,14). However, the resolution for noninvasive modalities is improving, and CT or MR angiography may provide additional information regarding the vessel wall that conventional angiography does not. Specifically, CT angiography may enable visualization of more of the distal branches of the mesenteric arteries than MR angiography, but MR angiography may be preferred in certain clinical situations (e.g., need to avoid iodinated contrast). In patients with a negative CT or MR angiogram result with a high degree of suspicion for abdominal involvement, it is reasonable to consider conventional angiography.
Recommendation: For patients with a history of severe PAN with abdominal involvement who become clinically asymptomatic, we conditionally recommend follow-up abdominal vascular imaging.
Follow-up imaging permits assessment of disease control and treatment response. In the view of the Voting Panel, follow-up imaging is particularly important when baseline imaging demonstrates aneurysmal disease. The timing of follow-up imaging is dependent, in part, on clinical factors, such as the extent and severity of vascular abnormalities, overall disease course, and response to therapy.
However, indefinite routine vascular imaging should be avoided if the abdominal vascular disease is shown to be quiescent.
Recommendation: For patients with suspected PAN involving the skin, we conditionally recommend obtaining a deep-skin biopsy specimen (i.e., a biopsy reaching the medium-sized vessels of the dermis) over a superficial skin punch biopsy to aid in establishing a diagnosis.
Indirect evidence (found in nonrandomized studies or studies in which findings were not primary aims) suggests that evaluation of deeper tissue is more effective at establishing a diagnosis of PAN (15,16), since a deeper-tissue sample is more likely to capture a medium-sized vessel. A deep-skin biopsy can be performed by a dermatologist as a deep (or “double”) punch biopsy and does not necessarily require invasive resection. This recommendation had strong support from the Voting Panel but remains conditional due to limited evidence.
Recommendation: For patients with suspected PAN and peripheral neuropathy (motor and/or sensory), we conditionally recommend obtaining a combined nerve and muscle biopsy over a nerve biopsy alone to aid in establishing a diagnosis.
Several studies suggest an increased yield with nerve and concurrent muscle biopsy as opposed to nerve biopsy alone (15–19). However, the biopsy should sample involved tissue and not be performed “blind” (i.e., sampling tissue that does not appear to be clinically affected). Of note, biopsy of an affected purely sensory nerve (e.g., sural nerve) is favored to avoid motor deficits.
Recommendation: For patients with a history of peripheral motor neuropathy secondary to PAN, we conditionally recommend serial neurologic examinations instead of repeated lectromyography/nerve conduction studies (e.g., every 6 months) to monitor disease activity.
This recommendation is based on the opinion of the Voting Panel due to a lack of published evidence addressing the issue. Repeated electromyography in a patient with stable symptoms is not recommended due to the invasive nature of this study. However, repeated electromyography/nerve conduction study would be warranted if there were uncertainty as to whether a new (or worsening) process was developing.
Recommendation: For patients with newly diagnosed active, severe PAN, we conditionally recommend initiating treatment with intravenous (IV) pulse glucocorticoids over high-dose oral glucocorticoids.
In several single-arm and comparative studies, evaluations of medical therapy were confounded by the use of other medications and did not control for IV pulse or high-dose oral glucocorticoid use (20–22). However, for active and severe disease specifically, patients may benefit from the additional mechanism of action of high-dose pulse glucocorticoids. That is, glucocorticoids may rapidly alter cell membrane and receptor function to promote suppression of inflammation once the glucocorticoid receptor is saturated (23). The Voting Panel noted that this recommendation was focused on patients with active, severe disease. For many patients with disease that is not associated with life-threatening manifestations (such as immediate risk of visceral infarct), oral glucocorticoids would be preferred due to lower overall glucocorticoid burden. For pediatric patients, pulse glucocorticoid therapy in other systemic immune disorders appears to have a favorable side-effect profile and is not more strongly associated with infections or other morbidities compared to oral glucocorticoids (24).
Recommendation: For patients with newly diagnosed active, severe PAN, we conditionally recommend initiating treatment with cyclophosphamide and high-dose glucocorticoids over high-dose glucocorticoids alone.
In newly diagnosed severe PAN, a single observational study and indirect evidence suggest that the use of cyclophosphamide has more benefits than glucocorticoid therapy alone, with no differences seen between oral and IV cyclophosphamide (25,26). Moreover, the use of additional cyclophosphamide cycles may provide a medium-term protection (3 years) against disease relapse, although this benefit wanes by 10 years (21). Use of cyclophosphamide may mitigate glucocorticoid toxicity by decreasing the cumulative steroid dose (27).
Recommendation: For patients with newly diagnosed active, severe PAN, we conditionally recommend initiating treatment with cyclophosphamide and glucocorticoids over rituximab and glucocorticoids.
While case reports have recently raised the question about the efficacy of rituximab use in PAN (28–30), its efficacy in PAN remains uncertain due to the lack of comparative or large single-arm studies in this disease.
Recommendation: For patients with newly diagnosed active, severe PAN who are unable to tolerate cyclophosphamide, we conditionally recommend treating with other nonglucocorticoid immunosuppressive agents and glucocorticoids over glucocorticoids alone.
Indirect evidence (i.e., data obtained from secondary outcomes in prior trials [25,31]) suggests that the combination of nonglucocorticoid immunosuppressive agents, such as azathioprine or methotrexate, with glucocorticoids is superior to glucocorticoids alone. Mycophenolate mofetil has not been well studied in PAN. No direct trials comparing glucocorticoid monotherapy with nonglucocorticoid combination therapy are available. In general, patients with severe PAN should be treated with cyclophosphamide over other immunosuppressive agents (26), but in patients unable to tolerate cyclophosphamide, another agent, such as azathioprine or methotrexate, is recommended over glucocorticoid monotherapy. Use of nonglucocorticoid immunosuppressive therapy may provide a glucocorticoid-sparing effect and minimize glucocorticoid toxicity, which is particularly significant in pediatric populations.
Recommendation: For patients with newly diagnosed active, nonsevere PAN, we conditionally recommend treating with nonglucocorticoid immunosuppressive agents and glucocorticoids over glucocorticoids alone.
In cases of nonsevere disease, a patient’s age, clinical condition, and their values and preferences are important factors in assessing treatment. Although some patients achieve disease remission while receiving glucocorticoids alone, a substantial number of patients ultimately require additional nonglucocorticoid therapy, usually azathioprine or methotrexate (20). This recommendation contradicts management recommendations based on the Five-Factor Score (32), in which patients without factors of severe disease can be treated with glucocorticoids alone. We favor the use of nonglucocorticoid therapy in nonsevere disease, since the addition of nonglucocorticoid therapy may minimize glucocorticoid use and subsequent toxicity.
Recommendation: In patients with newly diagnosed active, severe PAN, we conditionally recommend against using plasmapheresis combined with cyclophosphamide and glucocorticoids over cyclophosphamide and glucocorticoids alone.
In a single trial conducted in 1995, the use of plasmapheresis in PAN was evaluated, but a distinction between PAN and HBV-associated PAN was not made (33). Confidence intervals in this study were very wide. Thus, evidence supporting the use of plasmapheresis in non–HBV-associated PAN is unavailable and the benefit unclear. Plasmapheresis may be considered in catastrophic cases unresponsive to the recommended aggressive immunosuppressive therapies and may have a role in the management of HBV-related PAN.
Recommendation: For patients with PAN in remission who are receiving nonglucocorticoid immunosuppressive therapy, we conditionally recommend discontinuation of nonglucocorticoid immunosuppressive agents after 18 months over continued (indefinite) treatment.
Evidence for this recommendation is based on a single study that was performed in 1979 (31). Although a significant number of patients with PAN have disease relapse, the majority experience monophasic disease (20). Indefinite treatment may therefore not be needed. Disease needs to be in sustained remission (Table 1) before discontinuing therapy.
Ungraded position statement: The optimal duration of glucocorticoid therapy for PAN (e.g., tapering off by 6 months or longer than 6 months) is not well established, and thus, the duration of therapy should be guided by the patient’s clinical condition, values, and preferences.
In PAN, studies to determine the optimal length of time for glucocorticoid use have not been performed. In studies of other types of vasculitis (34), faster tapers led to more flares, which were often not organ-threatening and may have been mild. The Patient Panel preferred a longer taper, as a primary concern was disease control rather than glucocorticoid toxicity. Thus, duration of glucocorticoid use should be influenced by the patient’s clinical condition, values, and preferences.
Recommendation: For patients with severe PAN that is refractory to treatment with glucocorticoids and nonglucocorticoid immunosuppressive agents other than cyclophosphamide, we conditionally recommend switching the nonglucocorticoid immunosuppressive agent to cyclophosphamide over increasing glucocorticoids alone.
Based on the effectiveness of cyclophosphamide in new-onset severe PAN (26), indirect evidence suggests that cyclophosphamide should be used in patients with PAN that has evolved from a nonsevere presentation to one that is severe and does not adequately respond to other immunosuppressive agents.
Recommendation: For patients with newly diagnosed PAN who have achieved disease remission with cyclophosphamide, we conditionally recommend transitioning to another nonglucocorticoid immunosuppressive agent over continuing cyclophosphamide.
Due to its toxicity, cyclophosphamide therapy should not continue indefinitely and should generally be limited to 3–6 months per course (21). Based on the experience in antineutrophil cytoplasmic antibody–associated vasculitis, transitioning to another less toxic agent such as methotrexate or azathioprine is recommended once disease remission has been attained. Given the lack of clinical trials investigating remission maintenance in PAN, this recommendation was based on expert experience.
Recommendation: For patients with PAN with nerve and/or muscle involvement, we conditionally recommend physical therapy.
Indirect evidence for PAN is available for this recommendation from studies in inflammatory myositis. Based on this, we conditionally recommend this intervention due to its potential benefit and minimal risk. Physical therapy may be more beneficial for those with more substantial motor involvement. Patients on the Voting Panel expressed a high degree of enthusiasm for physical therapy as a modality for recovery and rehabilitation, in that they felt they had personally experienced benefit from physical therapy.
Recommendation: For patients with clinical manifestations of deficiency of adenosine deaminase 2 (DADA2), we strongly recommend treatment with tumor necrosis factor inhibitors over glucocorticoids alone.
DADA2 was first described in a series of patients with an early-onset (often childhood) PAN-like vasculitis (35). DADA2 is characterized by recurrent strokes and skin changes and diagnosed using ADA2 sequencing or ADA2 functional assays, and ADA2 mutations have been identified in patients diagnosed as having systemic PAN (36). Although only 1 case series has been published, the strong signal of benefit of tumor necrosis inhibitors provides evidence that treatment with tumor necrosis inhibitors, instead of conventional immunosuppressive agents such as cyclophosphamide, prevents strokes (35,37). Thus, physicians should consider DADA2 in the setting of a PAN-like syndrome with strokes, and if confirmed, we strongly recommend use of tumor necrosis factor inhibitors. The Voting Panel voted for a strong recommendation despite the small number of cases, stressing the prevention of severe adverse events.

