Cell Therapy
What is cell therapy?
Last Updated on May 16, 2025
Cell therapy is a type of immunotherapy that uses your body’s own modified cells to fight disease.
Cell therapies have been used in the treatment of some forms of cancer since 2002 and are being explored as a treatment option for some autoimmune diseases including vasculitis.
The first FDA approved clinical trials for cell therapy for the treatment of autoimmune disease were in 2017.
How could cell therapy be used to treat vasculitis?
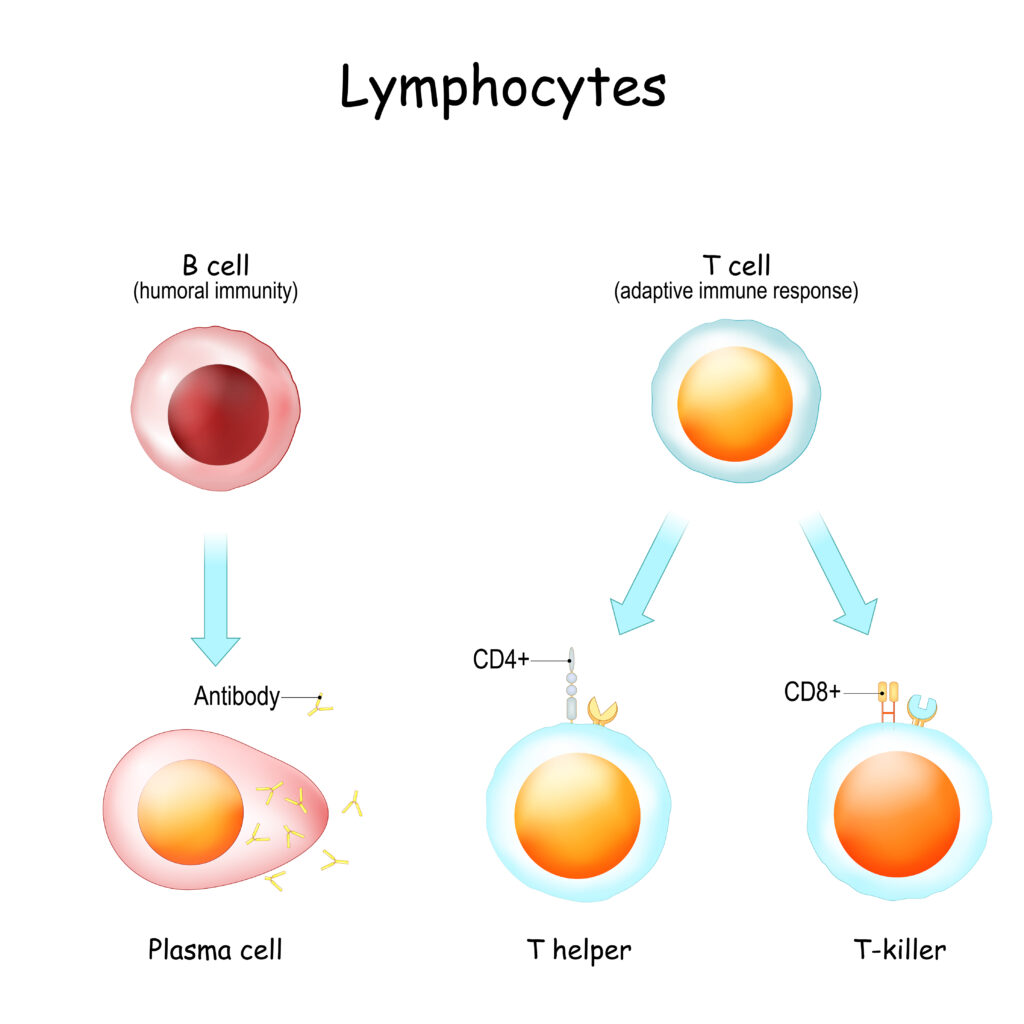
Your immune system is your body’s defense system. It is made up of billions of cells that have different jobs. Lymphocytes are one of the types of cells that make up your immune system. Lymphocytes are a type of white blood cell.
There are three types of lymphocytes.
- B-cells make antibodies that fight infections. In an immune system that is functioning normally, these antibodies do a great job of protecting you against bacteria and viruses. In some autoimmune diseases, something has triggered the B-cells to make antibodies against a portion of yourself (autoreactive).
- T-cells have many jobs in the immune system and there are different subtypes of T-cells that do each job.
- Helper T-cells (CD4+) help B-cells make antibodies to fight infection.
- Cytotoxic T-cells (CD8+) kill infected cells.
- Regulatory T-cells help control the immune response including controlling inflammation.
- Memory T-cells remember past infections so your immune system can respond more quickly the next time.
- Natural Killer (NK) cells kill infected or cancerous cells.
In some types of vasculitis, a person’s B-cells have become autoreactive meaning that instead of only producing antibodies that fight foreign bacteria, viruses or cancerous cells the B-cells are also reacting against the body’s own healthy tissue or cells, producing antibodies that cause damage and lead to autoimmune disease. With cell therapy, T-cells are genetically modified to find and destroy B-cells. The aim is to eliminate the B-cells that produce harmful autoantibodies. The hope is that once these B-cells are eliminated, your immune system will reset and the new B-cells produced by your immune system will not be autoreactive.
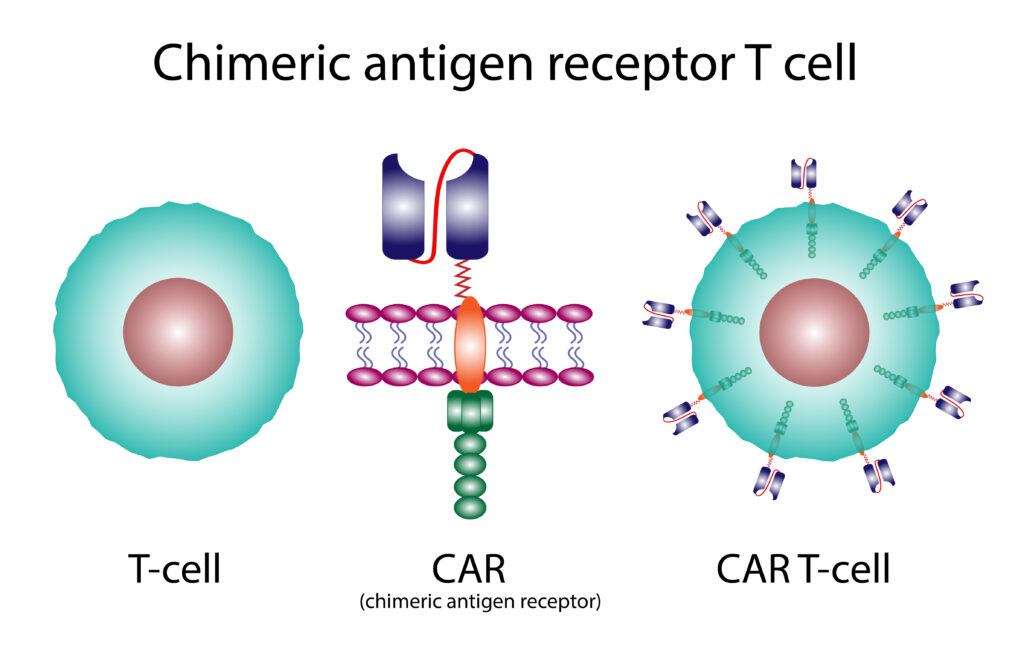
What's in a name?
In cell therapy the name of the therapy can be a clue as to which cells in your immune system are being modified. When your body’s T-cells are modified to fight disease, it is called CAR T-cell therapy. In CAR NK-cell therapy your body’s NK (natural killer cells) are the cells that are being modified.
The CAR in cell therapy stands for Chimeric Antigen Receptor. Chimeric means these cells are artificially engineered (produced in a lab) not naturally occurring. Antigen receptors are molecules primarily found on the surface of B and T lymphocytes that enable these cells to recognize and bind to specific antigens (foreign substances that trigger an immune response).
When designed to target only autoreactive cells such as those found in autoimmune diseases, cell therapy is sometimes called CAAR which stands for Chimeric AutoAntibody Receptor.
This is a general overview of the autologous cell therapy treatment process. The specific steps and procedures can vary depending on the type of cell therapy. In autologous cell therapy your own cells are modified and given back to you.
There is another cell therapy process called allogeneic cell therapy. In allogeneic cell therapy the cells come from someone else and have been stripped of anything seen as foreign. This reduces the time it takes to prepare the modified cells.
- Cell Collection Specific cells such T-cell or NK-cells are collected from your blood. This is done with a special machine that separates out the cells while the rest of your blood is returned to your body.
- Cell Modification The cells are sent to a specialized facility where they are genetically modified.
- Cell Expansion The cells are grown in the lab creating a large number of these specialized cells. This process takes several weeks.
- Patient Preparation You may be given medications to help suppress your immune system. This can improve the effectiveness of cell therapy.
- Modified Cell Infusion The modified cells are returned to your blood through an infusion.
- Monitoring and Follow-up After the infusion, you will be closely monitored for side effects and to assess the effectiveness of the cell therapy.
- Location Matters Cell therapy is not a treatment that can be received anywhere at any time. It is only available at specialized medical centers and requires extensive pre-treatment preparation and post-treatment monitoring. You and a dedicated caregiver must live close to (or be able to relocate for several months to an area close to) the medical center where the trial is being held.
- Protect Yourself Your ability to fight infection will be significantly decreased during your cell therapy treatment. You and your caregiver will need to take extra precautions to protect against and prevent infections during the treatment period.
- It Takes Time The process of collecting, modifying, and growing cells takes time. You will also need to be closely monitored for some time after the treatment to watch for treatment side-effects or adverse reactions and signs of infection.
It is important to note that cell therapy is in the early stages of research and clinical trials, and its long-term benefits and risks are still being studied.
What are the potential benefits of cell therapy?
- Increased Specificity Cell therapy could be designed to target only the specific autoreactive B-cells that are causing the autoimmune disease. Traditional immunosuppressants suppress the entire immune system, including healthy immune cells. This increased specificity could potentially reduce the risk of infections and other side effects.
- Potential for Long-Term Remission or Cure By eliminating the autoreactive B cells, cell therapy may reset the immune system so that it is no longer reacting against self-antigens. This could potentially lead to long-term remission or even a cure for some autoimmune diseases. Traditional immunosuppressants typically only manage the disease and require ongoing use.
- Reduced Side Effects Because cell therapy is more targeted, it may cause fewer side effects compared to broad-spectrum immunosuppressants that target many parts of the immune system. Traditional immunosuppressants can cause a range of side effects, including increased risk of infections, organ damage, and even cancer.
- One-Time or Limited Treatment In some cases, cell therapy may require only a single infusion or a limited number of treatments, whereas traditional immunosuppression often requires long-term or even lifelong medication.
What are the potential risks of cell therapy?
- Cytokine storms When many cells are being targeted and killed there is a release of proteins that can stir up a potentially dangerous inflammatory response.
- ICANS – Immune Effector Cell-Associated Neurotoxicity Syndrome The exact cause of ICANS is not known. It is believed that in rare cases activation of T-cells leads to leakage of immune cells in the brain causing Inflammation in the brain and neurotoxicity (damage to the nervous system).
- Anaphylaxis (Life-threatening Allergic Reaction) As with any treatment or medication there is a chance you could have a severe allergic reaction to the treatment.
- B-cell Aplasia Most cell therapies target all B-cells, not just autoreactive B-cells. This means if the cell therapy is successful you will have extremely low B-cell levels which lowers your body’s ability to make the antibodies that protect against infection.
- Infection As a result of the immune suppression you may receive to prepare your body for the infusion of the modified cells and the initial depletion of B-cells as the modified cells target your B-cells, you are at a higher risk for infection during the early phases of cell therapy treatment.
- Cancer Secondary cancers have been reported in a small number of patients who received cell therapy for treatment of their B-cell lymphomas (a type of cancer). When these cancerous cells were genetically sequenced, they contained genes from the modified cells used in the patient’s cell therapy treatment. This means that the modified cells had turned cancerous in a small number of patients who received cell therapy.
Patients receiving cell therapy are closely monitored for these and other potential side effects and complications.
Each trial has its own criteria for eligibility, but here are some general guidelines:
- The type of vasculitis you have may influence whether or not you are eligible to participate in a cell therapy trial. Current cell therapy clinical trials are testing therapies that target B-cells. Not all types of vasculitis are characterized by B-cell regulated autoantibodies.
- If you have certain damage to vital organs such as heart, kidneys, or lungs you may not be eligible.
- You will most likely not be able to participate if you have a current infection since cell therapy treatment makes it harder for your body to fight infection.
- Your age may determine whether or not you are eligible. Most cell therapy trials are for adult patients only and do not include pediatric patients.
Clinical Trials
This is an exciting time for vasculitis research with multiple active clinical trials for potential new treatments. Learn about CAR-T trials and NCT06868290 clinical trial for severe active GPA and MPA vasculitis.
Discussion Guide
Are you considering participating in a cell therapy clinical trial? Use these questions to help guide your discussion with the clinical trial team. CAR-T therapy could help reshape your immune system.
